[
The article was written by Guanao Yan, Ph.D. student of Statistics and Data Science at UCLA. Guanao is the first author of the Nature Communications review article [1].
Spatially resolved transcriptomics (SRT) is revolutionizing Genomics by enabling the high-throughput measurement of gene expression whereas preserving spatial context. Not like single-cell RNA sequencing (scRNA-seq), which captures transcriptomes with out spatial location data, SRT permits researchers to map gene expression to specific areas inside a tissue, offering insights into tissue group, mobile interactions, and spatially coordinated gene exercise. The rising quantity and complexity of SRT knowledge necessitate the event of strong statistical and computational strategies, making this discipline extremely related to knowledge scientists, statisticians, and machine studying (ML) professionals. Strategies reminiscent of spatial statistics, graph-based fashions, and deep studying have been utilized to extract significant organic insights from these knowledge.
A key step in SRT evaluation is the detection of spatially variable genes (SVGs)—genes whose expression varies non-randomly throughout spatial areas. Figuring out SVGs is essential for characterizing tissue structure, purposeful gene modules, and mobile heterogeneity. Nevertheless, regardless of the speedy growth of computational strategies for SVG detection, these strategies differ broadly of their definitions and statistical frameworks, resulting in inconsistent outcomes and challenges in interpretation.
In our current evaluation revealed in Nature Communications [1], we systematically examined 34 peer-reviewed SVG detection strategies and launched a classification framework that clarifies the organic significance of various SVG varieties. This text supplies an summary of our findings, specializing in the three main classes of SVGs and the statistical rules underlying their detection.
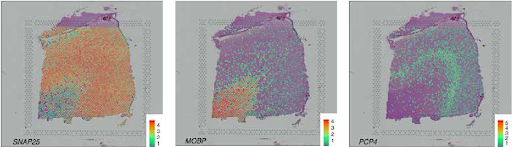
SVG detection strategies goal to uncover genes whose spatial expression displays organic patterns quite than technical noise. Primarily based on our evaluation of 34 peer-reviewed strategies, we categorize SVGs into three teams: Total SVGs, Cell-Sort-Particular SVGs, and Spatial-Area-Marker SVGs (Determine 2).

Strategies for detecting the three SVG classes serve totally different functions (Fig. 3). First, the detection of general SVGs screens informative genes for downstream analyses, together with the identification of spatial domains and purposeful gene modules. Second, detecting cell-type-specific SVGs goals to disclose spatial variation inside a cell sort and assist establish distinct cell subpopulations or states inside cell varieties. Third, spatial-domain-marker SVG detection is used to search out marker genes to annotate and interpret spatial domains already detected. These markers assist perceive the molecular mechanisms underlying spatial domains and help in annotating tissue layers in different datasets.

The connection among the many three SVG classes will depend on the detection strategies, notably the null and various hypotheses they make use of. If an general SVG detection technique makes use of the null speculation {that a} non-SVG’s expression is impartial of spatial location and the choice speculation that any deviation from this independence signifies an SVG, then its SVGs ought to theoretically embody each cell-type-specific SVGs and spatial-domain-marker SVGs. For instance, DESpace [2] is a technique that detects each general SVGs and spatial-domain-marker SVGs, and its detected general SVGs have to be marker genes for some spatial domains. This inclusion relationship holds true besides in excessive eventualities, reminiscent of when a gene displays reverse cell-type-specific spatial patterns that successfully cancel one another out. Nevertheless, if an general SVG detection technique’s various speculation is outlined for a particular spatial expression sample, then its SVGs might not embody some cell-type-specific SVGs or spatial-domain-marker SVGs.
To know how SVGs are detected, we categorized the statistical approaches into three main kinds of speculation checks:
- Dependence Check – Examines the dependence between a gene’s expression stage and the spatial location.
- Regression Fastened-Impact Check – Examines whether or not some or all the fixed-effect covariates, as an example, spatial location, contribute to the imply of the response variable, i.e., a gene’s expression.
- Regression Random-Impact Check (Variance Element Check) – Examines whether or not the random-effect covariates, as an example, spatial location, contribute to the variance of the response variable, i.e., a gene’s expression.
To additional clarify how these checks are used for SVG detection, we denote Y as gene’s expression stage and S because the spatial areas. Dependence take a look at is essentially the most common speculation take a look at for SVG detection. For a given gene, it decides whether or not the gene’s expression stage Y is impartial of the spatial location S, i.e., the null speculation is:

There are two kinds of regression checks: fixed-effect checks, the place the impact of the spatial location is assumed to be fastened, and random-effect checks, which assume the impact of the spatial location as random. To elucidate these two kinds of checks, we use a linear combined mannequin for a given gene for example:

the place the response variable ( Y_i ) is the gene’s expression stage at spot ( i ), ( x_i ) ( epsilon ) ( R^p ) signifies the fixed-effect covariates of spot ( i ), ( z_i ) ( epsilon ) ( R^q ) denotes the random-effect covariates of spot ( i ), and ( epsilon_i ) is the random measurement error at spot ( i ) with zero imply. Within the mannequin parameters, ( beta_0 ) is the (fastened) intercept, ( beta ) ( epsilon ) ( R^p ) signifies the fastened results, and ( gamma ) ( epsilon ) ( R^q ) denotes the random results with zero means and the covariance matrix:

On this linear combined mannequin, independence is assumed between random impact and random errors and amongst random errors.
Fastened-effect checks study whether or not some or all the fixed-effect covariates ( x_i ) (depending on spatial areas S) contribute to the imply of the response variable. If all fixed-effect covariates make no contribution, then:

The null speculation

implies

Random-effect checks study whether or not the random-effect covariates ( z_i ) (depending on spatial areas S) contribute to the variance of the response variable VarYi, specializing in the decomposition:

and testing if the contribution of the random-effect covariates is zero. The null speculation:

implies

Among the many 23 strategies that use frequentist speculation checks, dependence checks and random-effect regression checks have been primarily utilized to detect general SVGs, whereas fixed-effect regression checks have been used throughout all three SVG classes. Understanding these distinctions is essential to choosing the appropriate technique for particular analysis questions.
Enhancing SVG detection strategies requires balancing detection energy, specificity, and scalability whereas addressing key challenges in spatial transcriptomics evaluation. Future developments ought to concentrate on adapting strategies to totally different SRT applied sciences and tissue varieties, in addition to extending assist for multi-sample SRT knowledge to boost organic insights. Moreover, strengthening statistical rigor and validation frameworks will probably be essential for guaranteeing the reliability of SVG detection. Benchmarking research additionally want refinement, with clearer analysis metrics and standardized datasets to offer sturdy technique comparisons.
References
[1] Yan, G., Hua, S.H. & Li, J.J. (2025). Categorization of 34 computational strategies to detect spatially variable genes from spatially resolved transcriptomics knowledge. Nature Communication, 16, 1141. https://doi.org/10.1038/s41467-025-56080-w
[2] Cai, P., Robinson, M. D., & Tiberi, S. (2024). DESpace: spatially variable gene detection through differential expression testing of spatial clusters. Bioinformatics, 40(2). https://doi.org/10.1093/bioinformatics/btae027
]
Source link